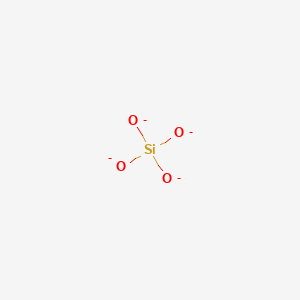
CH33SiH SiO44- Si2H6 SiOH4 SiF62-. Lewis dot structure of eqSiO_44 - eq contains Si which is bonded to 4 oxygen atoms.
Sio44 Lewis Structure - If you're looking for picture and video information related to the keyword you have come to pay a visit to the ideal blog. Our site provides you with suggestions for seeing the maximum quality video and image content, hunt and find more informative video content and images that match your interests. comprises one of thousands of movie collections from several sources, especially Youtube, therefore we recommend this video for you to see. You can also contribute to supporting this site by sharing videos and images that you enjoy on this site on your social media accounts such as Facebook and Instagram or tell your closest friends share your experiences concerning the ease of access to downloads and the information you get on this website. This blog is for them to visit this website.
Sio4 4 Lewis Structure Novocom Top
Placer les électrons autour du symbole de lélément chimique selon les quatre directions nord-sud-est-ouest.

Sio44 lewis structure. SiO4 YouTube 480x360 Sio4 4 Lewis Structure 320x180. Log Kow KOWWIN v167 estimate -432 Boiling Pt Melting Pt Vapor Pressure Estimations MPBPWIN v142. 4 All the solids Silicates contain silicate ion SiO4 4-as the basic structural unit.
28 - 12 16 electrons not in bonds. 16 2 8 lone pairs. Lewis and dot structure of NH42SO3 Al2 SO43 and Fe ClO43.
12 2 6 bonds. Structure and Formula of Silicates Chemistry Tutorial Key Concepts. 4 6 4 28 valence electrons.
A CH33SiH b SiO44 c Si2H6 d SiOH4 e SiF6 2 This problem has been solved. Etablir la structure de LEWIS. Before directly jumping into the lewis structure of SO2 lets have a quick discussion regarding the importance of lewis structure and the steps to draw it.
The basic building block the structural unit of silicate minerals is the SiO 4 4-tetrahedron. How many double bonds are there in one of the resonance structures. Write a Lewis structure for each of the following molecules and ions.
Electron Dot Diagrams Lewis Structures Atom and Covalent. Orthosilicate O4Si-4 CID 104812 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Write A Lewis Structure For Each Of The Following Molecules And Ions.
Draw the Lewis dot structure for SiO44-Si has 4 valence electrons O has 6 valence electrons the 4 give you 4 more electrons to work with. Look at it as O 3 Si-O-SiO 3 6- Answer 20. Déterminer le nombre délectrons de valence.
Sio4 4 Lewis Structure Pictures to Pin on Pinterest - PinsDaddy. Draw the Lewis dot formula for SiO 4 4- and then draw the Lewis dot formulas for two connected SiO 4 tetrahedra. So you have a total of 32 electrons.
40 - 28 12 shared electrons. 5 The silicate ion is tetrahedral in structure and when the one or more oxygen atoms between such tetrahedrons a. Carbonyl fluoride COF2 or CF2O CID 9623 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
Get the detailed answer. 8 5 40 electrons wanted. The structure of such anions is commonly described and depicted as consisting of silicon-centered tetrahedra connected by their vertices in such a way that each vertex is shared by at.
In the tetrahedron Si has an oxidation state of 4 and O has an oxidation state of -2. Draw the Lewis Structure for the following. Draw a Lewis structure for si044 which minimizes the formal charges.
In the SiO 4 4-tetrahedron each Si atom is covalently bonded to 4 oxygen atoms. Log Octanol-Water Partition Coef SRC. 64539 Adapted Stein.
SO2 Lewis Structure. You must include the when you have a charged atom or ion. Faire un tour si on a au plus quatre électrons à placer faire deux tours si on a plus de quatre électrons.
Create the structure from this. Lewis structure is the distribution of the electrons around the atoms of a compound. QUESTION 10 A molecule that has one double covalent bond is QUESTION 11 Based on the octet rule the most likely charge for an ion of Mg is- QUESTION 12 Based on the octet rule the most likely charge for an ion of N is.
Boiling Pt deg C. A quick explanation of the molecular geometry of SO4 2- including a description of the SO4 2- bond anglesLooking at the SO4 2- Lewis structure we can see th. This is the dot structure for SiO44-.
And yes 6 bonds means 2 double bonds are formed.
Sio4 4 Lewis Structure Novocom Top
Sio4 4 Lewis Structure Molecular Geometry Novocom Top
Draw A Lewis Dot Diagram For The Silicate Ion Sio44 Study Com
Orthosilicate O4si 4 Pubchem
Https Usflearn Instructure Com Courses 986898 Files 31845911 Download Wrap 1
Silicate Youtube
Orthosilicate O4si Chemspider
Https Usflearn Instructure Com Courses 986898 Files 31845911 Download Wrap 1
Http Employees Oneonta Edu Viningwj Chem241 Chem241 2014 Exam2 Answerkey Pdf